What is lacutamab?
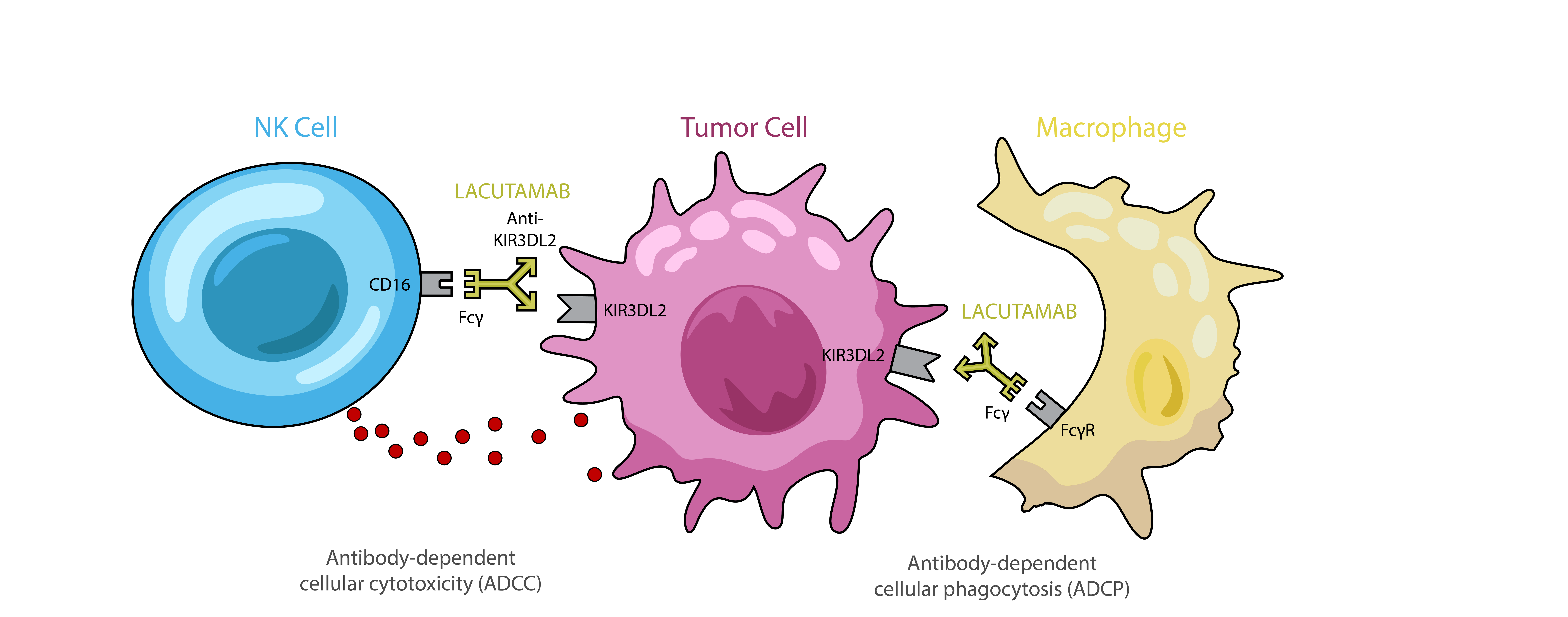
Lacutamab (IPH4102) is a first-in-class anti-KIR3DL2 humanized cytotoxicity-inducing antibody, which is currently in clinical trials for treatment of cutaneous T-cell lymphoma (CTCL), an orphan disease, and peripheral T cell lymphoma (PTCL).
European Medicines Agency (EMA) granted PRIME designation in November 2020 to lacutamab for the treatment of patients with relapsed or refractory Sézary syndrome who have received at least two prior systemic therapies.
Lacutamab has been granted orphan drug status in the European Union and in the United States for the treatment of CTCL and has been granted U.S. FDA Breakthrough Therapy Designation for relapsed or refractory Sézary syndrome.
Mechanism of action
Cutaneous T lymphomas
Cutaneous T lymphomas are lymphomas characterized by an abnormal accumulation of T lymphocytes, a type of white blood cell, in the skin and blood. This is an orphan indication, revealed by itching, plaques or nodules on the skin. Sometimes, skin and blood are affected concomitantly, as in the case of extensive mycosis fungoides or Sézary syndrome. Cutaneous T lymphomas occur more frequently in the elderly.
- Sézary syndrome is a rare form of cutaneous T-cell lymphoma. Symptoms of Sézary syndrome typically include severe rash and itching, rashes in the form of red, scaly and sometimes thick patches on the skin. Sézary syndrome is a rare disease, accounting for 5% of all cutaneous lymphomas.
- Mycosis fungoides is the most common subtype of cutaneous T lymphoma (CTCL), accounting for around half of all CTCLs. Its main symptoms are flat, scaly pink or red areas or spots on the torso, upper thighs or buttocks.
Peripheral T lymphoma is a type of non-Hodgkin's lymphoma (NHL) that originates in mature T lymphocytes and mainly affects the lymph nodes. However, it sometimes attacks other organs or tissues, such as bone marrow, skin, liver, spleen or organs of the digestive tract. Peripheral T lymphoma is an aggressive lymphoma, as it tends to progress rapidly. Most people are diagnosed when the disease is at an advanced stage (stage 3 or 4). Peripheral T-cell lymphoma accounts for approximately 15-20% of non-Hodgkin's lymphomas (NHL) and 5-10% of all adult malignant lymphomas.
Lacutamab is being investigated in mycosis fungoides, Sézary syndrome as well as in peripheral T-cell lymphoma.
- European Medicines Agency (EMA) granted PRIME designation in November 2020 to lacutamab for the treatment of patients with relapsed or refractory Sézary syndrome who have received at least two prior systemic therapies.
- The US Food and Drug Administration (FDA) granted Fast Track designation in January 2019.
- Granted orphan drug status in the European Union and in the United States for the treatment of CTCL.
| Lacutamab KIR3DL2 Proprietary program | TELLOMAK: global, open-label, multi-cohort Phase 2 clinical trial recruiting patients with Sézary syndrome and mycosis fungoides in the United States and Europe. Top-line results in mycosis fungoides were presented at ASCO Annual meeting 2024. Improved health-related quality of life data from TELLOMAK Phase 2 study in patients with cutaneous T cell lymphoma were presented at at the 66th American Society of Hematology (ASH) Annual Meeting.
| |||
| Preclinical | Phase 1 | Phase 2 | Phase 3 | |
KILT: randomized trial led by the Lymphoma Study Association (LYSA) to evaluate lacutamab in combination with chemotherapy GEMOX (gemcitabine in combination with oxaliplatin) versus GEMOX alone in patients with KIR3DL2-expressing relapsed/refractory PTCL. | ||||
| Preclinical | Phase 1 | Phase 2 | Phase 3 | |
Publications, posters and presentations